Medical devices
Accelerate innovation by 30% and ensure regulatory compliance with ISO, MDR, and FDA standards.

They trust us


Challenge
Medical device manufacturers must manage strict regulations (ISO 13 485, FDA 21 CFR Part 820, MDR, IDVR) that imposes strict traceability of product data and quality processes. The acceleration of innovation and the increasing complexity of product configurations require fluid collaboration between R&D, quality and production to ensure compliance by design and avoid errors.
Aletiq New Generation PLM makes it possible to centralize technical and regulatory data (DT, DHF, DMR, DHR) in a single source of truth to ensure compliance and accelerate innovation.

The single source of truth for your data and processes
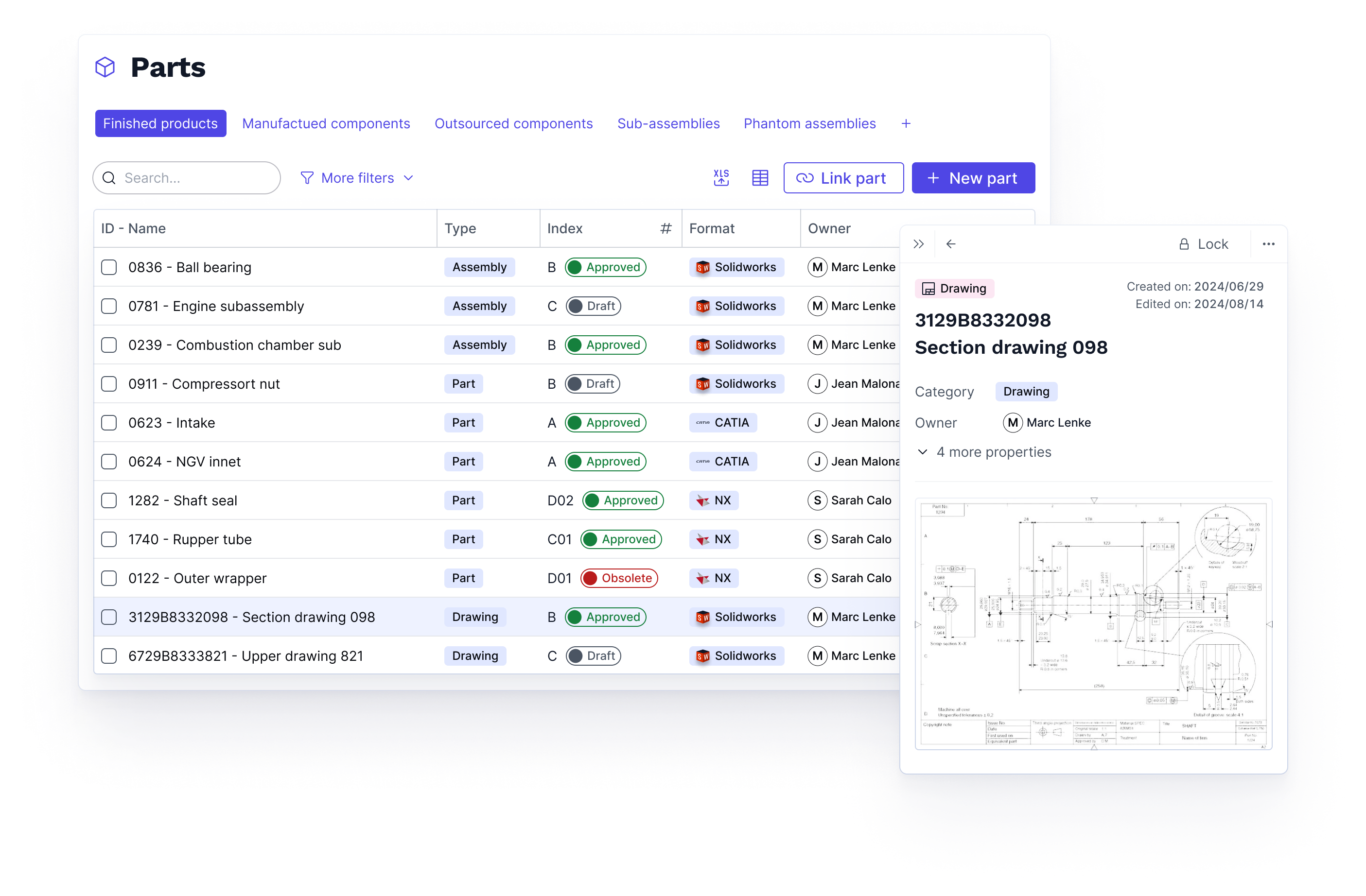




100%
compliance with ISO 13485, FDA Part 11, MDR, IDVR
-90%
time spent researching data
-30%
Time-to-Market
